Dialysis-medical RO system Feed-water (DRF) tests
Scope:

Physical & Sensory Parameters:
Colour (Appearance & True Colour Units), Odour, pH, Turbidity; & Electrical Conductivity (EC). And Total Dissolved Solids (TDS) by Gravimetry (TDSG).

Chemical Parameters:
Alkalinity, Total hardness of water (THW), Calcium (Ca) & Magnesium (Mg); Sodium (Na), Potassium (K), Nitrates (NO3); Sulphate (SO4), Iron (Fe) & Silica (SiO2).

Bacteriological Parameters:
MPNMost Probable Number, MPNMost Probable Number, MPN (Total coliforms),
Thermotolerant coliforms (TTC) & E. coli.
Total parameters: 6 + 10 + 3 = 19
Rationale:
Figure 1: Dialysis-medical Reverse Osmosis (RO) system – Feed water Tests (DRF)

Haemodialysis can expose the patient to more than 500L of water per week across the semi-permeable dialyser membrane. Average intake by healthy individuals is about 12-20L per week. Dialysis patients are exposed about 25 to 40 times the average intake of water. Hence the level of electrolytes, chemical contaminants and trace elements must be much lower than what is allowed for drinking water. Reverse osmosis (RO) helps reduce electrolytes, substantially removes many contaminants. Typically, RO treatment removes 90 to 99% of dissolved inorganic solids; and is a suitable treatment for safety of water used in dialysis. In other words, a good & fully functional RO system that removes a wide range of contaminants & minerals, is an important indicator of safe dialysis water. Considering the high volume of RO water consumed by dialysis centres, many hospitals and almost all dialysis centres have in house RO plants. Raw water for dialysis-medical RO plants may be sourced from borewells in campus, municipal water and/or tanker water.
RO purifiers are high pressure semipermeable membrane systems. Basic schema of a RO system consists of pre- and post-treatment units around the core RO unit. Pre-treated water is fed into RO unit under high pressure. Water that permeates through the RO membrane comes out as the product water, and the remaining concentrated water comes out as the reject water. The quality of feed water affects RO system performance and cost of operation. Color in feed water can irreversibly adsorb to the RO membrane and decrease the flow rate. Turbidity is a common indicatory of suspended particles. Reverse osmosis (RO) membranes are susceptible to three kinds of fouling, namely; (a) scaling, (b) colloidal fouling, and (b) biofouling (Ning, 2012). Scaling is usually due to various dissolved salts in the feed water. Test parameters like pH, alkalinity, and calcium provide inputs for computation of Langelier saturation index (LSI), which is an indicator of potential scaling. Colloidal fouling is usually due to heavy loads of suspended colloidal particles ranging from visible to invisible submicron particles, such as clays (aluminium silicate), silt (ferric-aluminium-magneisium silicates), silica (SiO2). Biofouling is usually due to growth and anchoring of microorganisms on the memberanes. Moderate temperatures and minimal nutrient levels in RO raw waters can support at times explosive growths of microorganisms. Bacteria capable of cell division every 20 minutes can grow from a normal count per unit volume of water to millions in the period of an 8 hours shift. Due to the tendency of bacteria to secrete polymers that anchor themselves to surfaces to facilitate growth as the biofilm, this fouling mechanism is unique and poses a serious threat to the operation of RO systems. In addition, some biofilms produce endotoxins increasing vulnerability for dialyisis relaated complications. Although RO membranes can remove virtually all microorganisms, it is currently recommended that only microbiologically safe (i.e., coliform negative) water be fed into RO systems, to minimise biofouling and risk of endotoxins. Hence, it is desirable to check for microbial characteristic (BCT & HPC) of feed water, so that appropriately sized disinfection unit can be incorporated into the pre-treatment stage (Ning, 2012).
Figure-2: Typical schema of a dialysis-medical RO plant
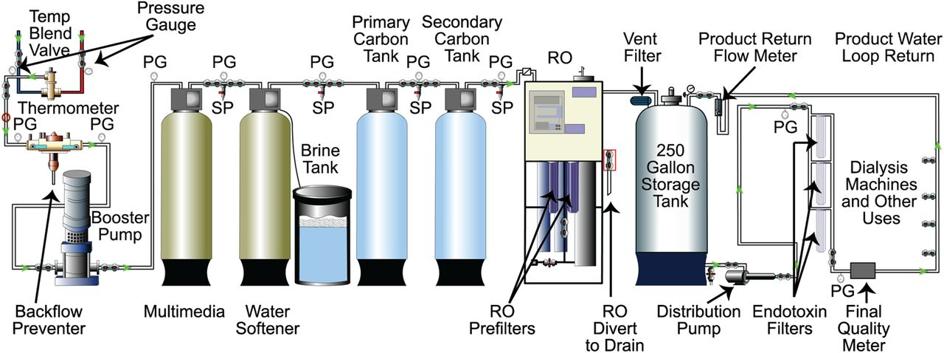 Source: Figure-2 in Kasparek and Rodriguez, What Medical Directors Need to Know about Dialysis Facility Water Management. CJASN, 2015 10(6):1061-1071. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4455220/
Source: Figure-2 in Kasparek and Rodriguez, What Medical Directors Need to Know about Dialysis Facility Water Management. CJASN, 2015 10(6):1061-1071. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4455220/
One key consideration in use of municipal supply as feed water for dialysis RO systems is chlorine. Free chlorine can damage the RO membrane, resulting in lower salt rejection and poor-quality permeate. Exposure to free chlorine at levels > 0.1mg/L for 200 to 1000 hours can permanently damage RO membranes. Moreover, combined chlorine (chloramines) is potentially toxic for dialysis patient, carrying risk of haemolytic anaemia. This is the rationale for the total chlorine limit prescribed in table-1 of ISO 23500-3. Chlorine and chloramines can pass through the majority of elements of the RO systems. Hence it is desirable to ensure that feed water for dialysis RO systems does not contain any significant amount of chlorine. Thus, if municipal water is used as feed water, it's chlorine level should be monitored and special arrangements made for removal of excess chlorine. Granular activated carbon can be used to remove chlorine and chloramines. As granular activated carbon can remove many other contaminants in addition to chlorine/chloramines, the pre-treatment unit should be adequately sized. Groundwater would be free of chlorine, unless chlorine-based disinfectants are added by way of pre-treatment. In any case, it is desirable to test for total chlorine to assure qualify of feed water for dialysis RO systems. Residual chlorine is to be tested onsite and is not included in the scope of this laboratory test package.
The IS 17646 (Part 3) : 2021 / ISO 23500 part 3 : 2019 specifies requirements of water for haemodialysis and related therapies (ISO, 2019). The IS 17646-3:2021 / ISO 23500-3:2019, requires that the feed water for any dialysis RO water system satisfies the requirements for potable water. This implies that feed water for dialysis-medical RO plants must first satisfy the Indian Standard drinking water specification (IS 10500:2012). Recognising that laboratory facilities and analytical tools to test for trace elements may not be easily available, and to minimise operational constraints, ISO 23500-3, provides for an alternative indicator, namely RO rejection rate. Similar alternative provisions for demonstration of compliance are contained in the UK guidelines (Hoenich, et al, 2016) and the South Australian Haemodialysis Guidelines (Whittington et al, 2015). In order to compute the RO rejection rate, both feed water and product water have to be analysed.
The scope of IHS Laboratory dialysis-medical RO feed water tests (DRF) include enough parameters to establish potability, assess vulnerability for fouling of RO membranes, and establish base line mineral content for estimation of RO rejection rates. It also takes into consideration the possibility that the feed water may be sourced from borewells (groundwater), municipal supplies (treated surface water) and tanker water which may be from borewells or municipal supply.
Sample - Collection, Storage & Transportation:
Refer methods of sampling specified in IS 3025 part 1 :1987 for chemical tests and in IS 1622 : 1981 for bacteriological tests. Sample should be representative of the water to be tested and should be collected with utmost care to ensure that no contamination occurs at the time of collection or prior to examination by the laboratory.
Step-1: Gather all that you need for collection of water sample:
One litre sample collected in a clean and dry clear or amber colour polypropylene bottle (CBWS/ABWS) is required for physical and chemical tests. About 250 ml of water sample collected in a sterile bottle with thiosulfate (SBT) is required for bacteriological tests. You need a pair of sample collection bottles (1xCBWS + 1xSBT), two black or dark colour polythene bags (small garbage bag will do) to minimise exposure of samples to sunlight, ice packs to keep the sample bottles cool during transport and a carry bag for convenient transport.
Step-2: Identify sampling point and time:
Identify the sump or ground level tank from which the RO plant draws its feed water. Locate sample collection tap either on pretreatment feeder pump delivery pipe or borewell water delivery pipe. If for some reason no sample collection tap is available/accessible from anywhere on the borewell delivery pipe line or pretreatment feeder pump delivery, the dip-samples from inside the sump would be the last resort. Choose a time when the laboratory will be open to receive the sample, allowing for required transportation time.
Step-3: Collect sample:
Wash both your hands with soap and water, wipe with a clean towel and let it dry. Request an assistant to wash his/her hands and standby. Label the sample collection bottles and place it within easy reach, but do not open at this stage. Have ice packs ready. Flush the delivery pipe by letting water out for, say 2-3 minutes. Do not touch the flow from delivery pipe. Collect sample for physical and chemical analysis first; then the bacteriological-sample. Hold the bottle in one hand and open and hold the cap in the other hand avoiding to touch inner side of the cap. Place opened bottle mouth under the delivery pipe or spout avoiding direct contact. As the bottle is about to be full, quickly remove the bottle away from water stream and replace cap tightly. Wipe outside of bottle dry with a clean and dry tissue or cloth. Place each bottle inside separate dark colour bags, tie ice packs around each of them and place it in a carry bag for transport to laboratory.
Step-4: Rush to laboratory as soon as possible. Do not store!
Information About Source, Context, Intended Use & Concerns:
Provide as much detail as you can about the supplier, batch number, bottling date, date of opening of bottle, etc. Mention about intended use of the water, the reason why you are ordering the test, as well as doubts and concerns, if any. These information help in interpretation of test results. Occasionally, the IHS Laboratory may contact you for clarifications and additional information about the source and its environment, to help interpretation of test results.
Test Method & Duration:
Physical and chemical characteristics of water sample are tested according appropriate parts of the IS3025 and/or American Public Health Association (APHA). For bacteriological analysis methods specified in IS1622 of 1981 are used. Depending on duration of bacteriological analysis, and gathering of additional information for interpretation of results; report will be available in 3 to 5 days.



To pick up sample collection bottle and/or schedule collection of samples: Email: ihslab@ihs.org.in with your address and contact telephone; OR WhatsApp: +919848011251; Or Call: 23211013/4.
References:
- Hoenich Nic; Mactier Robert; Boyle Gerard; et al. Guideline on water treatment facilities and water quality for haemodialysis and related therapies. UK Renal Assoc. & Assoc. of Renal Technologists; 2016. https://www.renaltech.net/uploads/1/3/6/4/136400/guideline_on_water_treatment_systems_dialysis_water_and_related_therapies__jan_2016.pdf
- IS 10500. Indian Standard Drinking Water Specification. Second Revision. New Delhi: Bureau of Indian Standard (BIS); 2012 May. https://law.resource.org/pub/in/bis/S06/is.10500.2012.pdf
- IS 1622: 1981. Indian Standard Methods of Sampling and Microbiological Examination of Water. New Delhi: Bureau of Indian Standard (BIS); Indian Standard, IS1622 - 1981, Reaffirmed 1996, Amended 2003. https://law.resource.org/pub/in/bis/S02/is.1622.1981.pdf
- IS 3025 Part 1: 1987. Methods of Sampling and Test (Physical and Chemical) for Water and Wastewater. Part 1 Sampling. New Delhi: Bureau of Indian Standard (BIS); Indian Standard. Reaffirmed 2003. https://law.resource.org/pub/in/bis/S02/is.3025.01.1987.pdf
- IS 17646 (Part 3) : 2021 Preparation and quality management of fluids for haemodialysis and related therapies Part 3 Water for haemodialysis and related therapies. Identical under dual numbering (ISO 23500-3 : 2019).
- ISO 23500-3. 2019. Preparation and quality management of fluids for haemodialysis and related therapies — Part 3: Water for haemodialysis and related therapies. Geneva, Switzerland: International Standards Organization (ISO); 2019 Feb; ISO 23500-3:2019.
- Kasparek Ted and Rodriguez Oscar E. What Medical Directors Need to Know about Dialysis Facility Water Management. Clinical Journal of the American Society of Nephrology. 2015 Jun 5; 10(6):1061-1071.https://pubmed.ncbi.nlm.nih.gov/25979976/
- Ning Robert Y. Chemistry in the Operation and Maintenance of Reverse Osmosis Systems. Chapter 4 in: Ning Robert Y. Advancing Desalination. London: Intech Open; 2012 Sep 14; pp. 85-95.https://www.intechopen.com/chapters/37328
- Whittington Tiffany; Donnelly Fiona; Passaris George; et al. South Australian Haemodialysis Guidelines: Routine Water Testing and Reverse Osmosis Monitoring. 2015 Jun.https://www.sahealth.sa.gov.au/wps/wcm/connect/bd096e004a4622f78af7cfb0cfc4074a/Haemodialysis_Routine+Water+Testing+and+Reverse+Osmosis+Monitoring_V3_1.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-bd096e004a4622f78af7cfb0cfc4074a-og8xKiH
- WHO. 2011. Guidelines for drinking-water quality. Fourth Edition. Geneva: 2011. https://www.who.int/publications/i/item/9789241549950
