Surface water Basic Profile (SBP) Tests
Scope:

Physical & Sensory Parameters:
Colour (Appearance & True Colour Units), Odour, pH, Turbidity; Total Suspended Solids (TSS), Electrical Conductivity (EC) & Total Dissolved Solids (TDS) by Gravimetry (TDSG).

Chemical Parameters:
Total hardness of water (THW), Calcium (Ca) & Magnesium (Mg); Ammoniacal-Nitrogen (NH3/4-N), Nitrites (NO2) & Nitrates (NO3); Chlorides, Sulfate, Phosphate (PO4), Sodium (Na), Potassium (K), Total Oil & Grease (TOG). Computation of Sodium Adsorption Ratio (SAR).

Bacteriological Parameters:
MPNMost Probable Number, MPNMost Probable Number, MPN (Total coliforms),
Thermotolerant coliforms (TTC) & E. coli.
Note: the biological parameters are same as in BCT.
Total parameters: 7 + 12 + 3 = 22 + 1 Estimated ratio (SAR)
Rationale:

Surface water is any body of water above ground, including streams, rivers, reservoirs, lakes, tanks, and ponds . Most water utilities source raw water from surface water bodies. For example; the Hyderabad metro water supply is sources from reservoirs built across several rivers, namely; Musi, Manjeera, Krishna and Godavari. After satisfying drinking and domestic water supply needs, surface water is used for several important purposes, such as irrigation, recreation, fishery, etc. Pristine water stored in a protected reservoir would be a good aquatic habitat, useful for fishery, suitable for irrigation and , can be used for drinking with minimal treatment. In addition, water reservoirs provide excellent opportunities for recreation, by way of a scenic water front, healthy for bathing and swimming, or for that matter sailing, boating etc.
Dynamic interaction of atmosphere, rainfall pattern, and geochemical conditions of drainage basins are natural determinants of water quality characteristics of freshwater bodies. If surface waters were totally unaffected by human activities, up to 90-99 per cent of global freshwaters, would have natural chemical concentrations suitable for aquatic life and most human uses (Chapman, 1996). Continuous flow in streams and rivers and large area of surface water bodies do provide some natural regenerative capacity against pollution due to exposure to animal and human activities. However, various factors such as dynamic interaction of changes in natural flow, manmade obstructions, inadequate protection, encroachments, urban run-off, inadequately treated industrial effluents and many more factors can affect surface water bodies, which in turn may affect suitability for various intended use. The Central Pollution Control Board uses the concept of designated-best-use for monitoring and assessment of water quality in surface water bodies. A particular water body may have several uses and the quality criteria for each would vary. For any given water body meant for several purposes, the use which demands highest quality of water is called its designated-best-use. Five quality classes are identified adopting a minimal set of primary water quality criteria. (Table 1).
Many industries that require ultra-refined water in manufacturing need RO product water. Pure water is essential for pharmaceutical manufacturing. All pharmacopeias require pharma manufacturers to begin with drinking water and purify it further to standards depending on the use, such as purified water or water for injection. RO water is used as bulk water for injection (BWFI) and for the preparation of parenteral solutions. RO product is used as type-3 water in laboratories for cleaning of glass wares, in water baths, autoclaves, and as feed water for type-1 or type-2 deionized water.
In SBP we have packaged a basic set of physical, chemical and bacteriological parameters, for routine monitoring of surface water quality to determine prima-facie suitability for various common purposes and sources of pollution, if any. The natural acid-base balance of a water body can be affected by industrial effluents and atmospheric deposition of acid-forming substances. Changes in pH can indicate the presence of certain effluents, particularly when continuously measured and recorded, together with the electrical conductivity. Excess of TSS, and turbidity affects fishery, suitability as a source of drinking water, bathing and recreational use, and may be due to impact of untreated sewage, urban run-off, agricultural activities in the foreshore, and certain industrial effluents. EC and TDS indicate salinity, which is an important consideration for irrigation & hydroelectric power plants. Hazardous chemical waste, food processing, textile, chemical and pharmaceutical effluent, can substantially increase EC and TDS.
| Quality Class | Designated Best Use | Primary Water Quality Criteria | |||
|---|---|---|---|---|---|
| pH | Dissolved Oxygen | Biochemical O2 Demand | MPN (Total Coliforms) | ||
| A | Drinking water source without any treatment, except for chlorination. | 6.5 to 8.5 | ≥ 6 mg/L | ≤ 2 mg/L | ≤ 50 cfu / 100 ml |
| B | Organised outdoor bathing | 6.5 to 8.5 | ≥ 5 mg/L | ≤ 3 mg/L | ≤ 500 cfu / 100 ml |
| C | Drinking water source with conventional treatment | 6.5 to 8.5 | ≥ 4 mg/L | ≤ 3 mg/L | ≤ 5000 cfu / 100 ml |
| D | Propagation of wildlife and fisheries | 6.5 to 8.5 | ≥ 4 mg/L | Ammonia ≤ 1.2 mg/L | |
| E | Irrigation, industrial cooling, and controlled disposal. | 6.5-8.5 | EC ≤ 2250 µS/cm; Sodium Adsorption Ratio < 26; Boron < 2 mg/L | ||
| Source: Based on Table 1 in CPCB. 2008. Guidelines for Water Quality Management. Government of India, Ministry of Environment & Forests - Central Pollution Control Board (CPCB), New Delhi. | |||||
Unpolluted waters contain small amounts of ammonia. Higher concentrations could be an indication of organic pollution such as from domestic sewage, industrial waste and fertilizer run-off. Ammonia is, therefore, a useful indicator of organic pollution. Determination of nitrate plus nitrite in surface waters gives a general indication of the nutrient status and level of organic pollution. Phosphorus is an essential component of the biological cycle in water bodies. High concentrations of phosphates can indicate the presence of pollution and are largely responsible for eutrophic conditions. The management of a lake or reservoir, particularly for drinking water supply, requires a knowledge of the levels of phosphate in order to help interpret the rates of algal growth. Higher levels of chloride is often an indicator of sewage discharges in to water bodies.
Sodium is commonly measured where the water is to be used for agricultural purposes, particularly irrigation. Elevated sodium in certain soil types can degrade soil structure thereby restricting water movement and affecting plant growth. The sodium adsorption ratio (SAR) is used to evaluate the suitability of water for irrigation. Calcium and magnesium values are required for computation of SAR. Potassium in natural waters is usually low. However, potassium salts are widely used in industry and in fertilizers for agriculture and enter freshwaters with industrial discharges and run-off from agricultural land.
Microbial indicator organisms provide a measure of faecal contamination and therefore the sanitary quality of the water body. Bacteriological analysis with the coliform tests help evaluate overall cleanliness, and level of faecal contamination of the water body.
This test package is suitable to monitor and evaluate quality of water in urban water bodies, lakes, reservoirs, rivers, streams, etc. Dissolved oxygen (DO), biochemical oxygen demand (BOD), and chemical oxygen demand (COD) are fundamental part of surface water quality assessment since oxygen is involved in, or influences, nearly all chemical and biological processes within water bodies. However, these tests require special care in sample collection, transportation and storage. Hence, included in a separate test package, namely; Surface water Oxygen Demand (SOD).
Sample - Collection, Storage & Transportation:
Follow methods of sampling specified in IS 3025 part 1 : 1987 for chemical tests and in IS 1622 : 1981 for bacteriological tests. Sample should be representative of the water to be tested and should be collected with utmost care to ensure that no contamination occurs at the time of collection or prior to examination by the laboratory.
Step-1: Gather all that you need for collection of water sample:
Two litre sample collected in a clean polypropylene can or bottle is required for physical and chemical tests. About 250 ml of water sample collected in a clean sterile bottle (CBS) is required for bacteriological tests. You need two 1 Litre clean dry polypropylene bottles and one 250 ml clean sterile bottle (2xCBWS + 1xCSB), two black or dark colour polythene bags (small garbage bag will do) to minimise exposure of samples to sunlight, ice packs to keep the sample bottles cool during transport and a carry bag for convenient transport.
Both CBWS and CSB are available from the IHS Laboratory. If it is not feasible for you to collect the specified sample collection bottles from the laboratory and you must collect samples, freshly emptied packaged drinking water bottles or new PET water bottles and cans may be used, in that order. If only one freshly emptied packaged water bottle is available, then use the same to collect bacteriological sample. Do not use empty beverage bottles such as ThumsUp, Maaza, Sprite. Nutrients and other residues in such bottles may promote growth of bacteria and bias test results.
Step-2: Identify sampling point and time:
Quality of water often varies in different parts of surface water bodies. Therefore, a single sampling-point is usually not enough. The number of sampling-locations and -depths depend on size of the water body and study objectives. However, a single sample coupled with contextual data can help determine prima-facie suitability for intended use and yield useful clues about possible sources of pollution, if any. If you plan to take a single sample, prefer the deeper ends, or clear areas where water is more than one meter. If feasible, approach the sampling point by a boat or from an accessible platform on water. Otherwise, slowly wade in towards the spot, up to about your waist deep, taking care to minimise disturbance of the bottom, so that you do not kick up any sediment to rise to surface. After reaching the sampling spot, wait for a few minutes for kicked up sediments around you to settle down. Then extend your arm to collect sample from a spot minimally affected by your entry. If this is not feasible, identify a spot on the bank from where you can draw water using a pole and a clean bucket.
For nalas, streams and rivers, identify a spot upstream of any bridge, culvert, crossing. Collect sample slightly below surface of flowing water, while avoiding bottom of the stream. If depth of stream permits, immerse the bottle completely about 4 inches deep. If stream is too shallow to immerse the bottle fully, collect as much as possible, being very careful not to touch the bottom where sediments can be disturbed and make sure no surface film flows into the bottle.
Step-3: Collect sample:
-
Always have an assistant to standby, just in case you need help as you wade into the waterbody or stream, to help draw water using etc. Both you and the assistant should wash both hands with soap and water, either at a distance from the intended sampling point, or by drawing a bucket of water to the bank for this purpose.
- Ready a clean & dry bucket, a suitable pole/rope if direct immersion is not feasible.
- Label the sample collection bottles and place within easy reach, but do not open at this stage. Have ice packs ready.
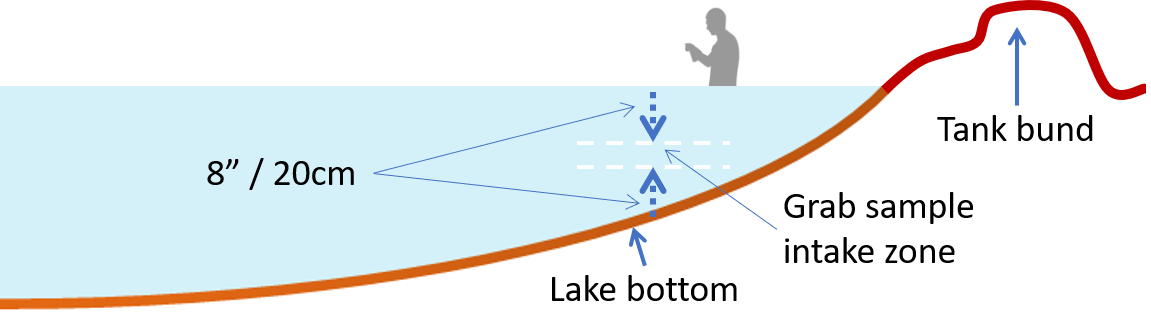
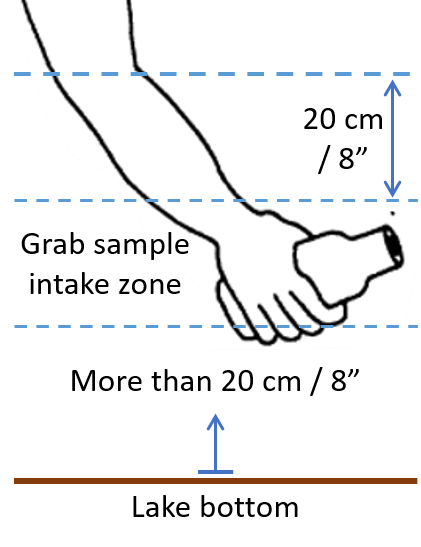
- Remember that mouth of the dipped bottle should not go too close to the lake bottom. The ideal depth zone for intake of grab sample would be at least 20 cm above lake floor and 20 cm below surface. Thus, the depth of water at the sampling point should be at least 2ft, so that there is about 10 cm of intake zone.
- Carefully wade into the lake and stop at a point where water is knee deep. Stand facing the lake. Stand still for some time, until kicked up sediments settle down.
- Face towards the lake centre (deeper area). You will need to bend forward to collect sample from a point that has not been disturbed by your entry into the lake.
-
 Immerse the open bottle, mouth down, slowly turn and allow water to slowly run into the bottle minimizing turbulence. If this is not feasible, use clean bucket and pole/rope to draw water and pour into sample collection bottles, close cap tightly.
Immerse the open bottle, mouth down, slowly turn and allow water to slowly run into the bottle minimizing turbulence. If this is not feasible, use clean bucket and pole/rope to draw water and pour into sample collection bottles, close cap tightly.
- Hold the bottle towards deeper part of the lake, tilt it downwards and plunge the bottle opening under the water surface, until your elbow touches water surface. Turn the submerged bottle gradually as your elbow makes 45° angle with respect to water surface, and the opening pointed away from you, towards deeper areas of the lake. Fill the bottle slowly, allowing air inside the bottle to escape gradually. As the bottle fills more and more, gradually turn the bottle up until its mouth looks up vertically upwards, while still under water.
- Put sample bottles in dark bags, wrap ice packs and place in carry bag.
Step-4: Transport to laboratory:
Preferably within 6 hours.
Step-5 Store sample, if required:
If immediate transport is not feasible, store it inside the regular chamber (not freezer) of a refrigerator until you are ready. Must transport within 24 hours from the time of collection.
Information About Source, Context, Intended Use & Concerns:
Provide as much detail as you can about the water body, sampling point, activities & environment around the sampling point. Click some photos and record GPS coordinates, if you can. Mention about intended use of the water, the reason why you are ordering the test, as well as doubts and concerns, if any. Occasionally, the IHS Laboratory may contact you for clarifications and additional information about the source and its environment, to help interpretation of test results.
Test Method & Duration:
Physical and chemical characteristics of water sample are tested according appropriate parts of the IS3025 and/or American Public Health Association (APHA). For bacteriological analysis methods specified in IS1622 of 1981 are used. Depending on duration of bacteriological analysis, and gathering of additional information for interpretation of results; report will be available in 3 to 5 days.



To pick up sample collection bottle and/or schedule collection of samples: Email: ihslab@ihs.org.in with your address and contact telephone; OR WhatsApp: +919848011251; Or Call: 23211013/4.
References:
- CPCB. 2008. Guidelines for Water Quality Management. Government of India, Ministry of Environment & Forests - Central Pollution Control Board (CPCB), New Delhi.
- Chapman Deborah. 1996. Water Quality Assessments - A Guide to Use of Biota, Sediments and Water in Environmental Monitoring. London: published on behalf of WHO by F & FN Spon. https://apps.who.int/iris/handle/10665/41850
- IS 1622: 1981. Indian Standard Methods of Sampling and Microbiological Examination of Water. New Delhi: Bureau of Indian Standard (BIS); Indian Standard, IS1622 - 1981, Reaffirmed 1996, Amended 2003. https://law.resource.org/pub/in/bis/S02/is.1622.1981.pdf
- IS 3025 Part 1: 1987. Methods of Sampling and Test (Physical and Chemical) for Water and Wastewater. Part 1 Sampling. New Delhi: Bureau of Indian Standard (BIS); Indian Standard. Reaffirmed 2003. https://law.resource.org/pub/in/bis/S02/is.3025.01.1987.pdf
