RO product water Profile (ROP) Tests
Scope:

Physical & Sensory Parameters:
Colour (Appearance & True Colour Units), Odour, Turbidity, pH, Electrical Conductivity (EC), & Total Solids-Dried.

Chemical Parameters:
Alkalinity, Total Hardness, Ca, Mg, NO3, Fluoride, Boron, Chloride, Sulfate, Iron, Manganese, & Sodium.

Bacteriological Parameters:
Detection of Total coliforms & E. coli in 100 ml by MF Or ESA. Aerobic Microbial Count (AMC).
Total parameters: 6 + 12 + 3 = 21
Rationale:
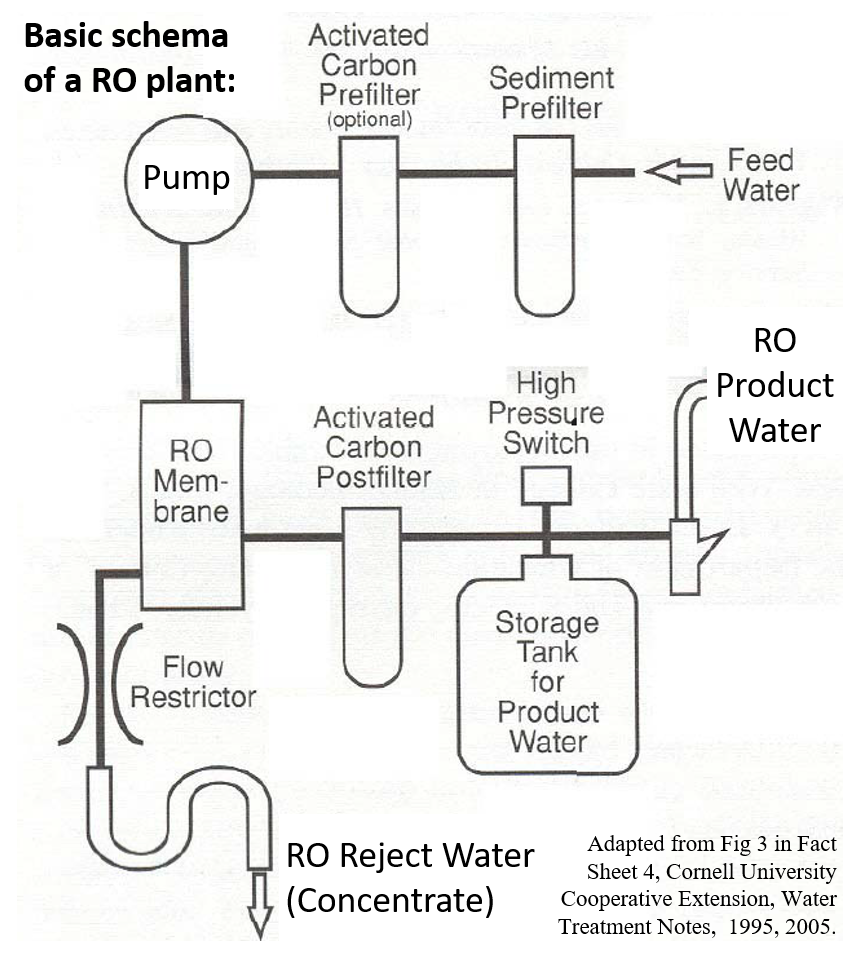
Reverse Osmosis (RO) technology is used extensively to produce water for various purposes. RO purifiers are high pressure semipermeable membrane systems. Basic schema of a RO system consists of pre- and post-treatment units around the core RO unit. Pretreated water is fed into RO unit under high pressure. Water that permeates through the RO membrane comes out as the product water, and the remaining concentrated water comes out as the reject water. The simplest pre-treatment would include a sediment filter to catch suspended solids and an activated carbon unit to adsorb volatile compounds. Depending on quality of feed water, additional pre-treatment units, such as antiscalant dosing systems, ultrafiltration units, would be included. Some feed waters The RO phase may include additional units for multiple passes, depending on feed water quality and/or special product water requirement. Post-treatment activated carbon units are provided to maximise adsorption of volatile organic compounds. Additional post-treatment units may be provided for permeate disinfection, conditioning, and corrosion control, depending on product water requirements.
High pressure coupled with small pore size (<0.001 micron) and electrical polarization enables RO systems to remove various dissolved solids, with up to 99.8% rejection rates. However, actual rejection rates vary depending on feed water quality, organic-inorganic composition of dissolved solids, RO system performance etc. RO membranes are vulnerable to scaling, colloidal- and bio-fouling, which affect performance and quality of product water. Even though RO product water is devoid of nutrients, gram-negative bacteria can multiply relatively fast, particular if there is stagnation in post-treatment lines (USFDA, 2014).
Many industries that require ultra-refined water in manufacturing need RO product water. Pure water is essential for pharmaceutical manufacturing. All pharmacopeias require pharma manufacturers to begin with drinking water and purify it further to standards depending on the use, such as purified water or water for injection. RO water is used as bulk water for injection (BWFI) and for the preparation of parenteral solutions. RO product is used as type-3 water in laboratories for cleaning of glass wares, in water baths, autoclaves, and as feed water for type-1 or type-2 deionized water.
Almost all bottled and bulk water producers in Hyderabad use RO technology. Growth of RO plants in Hyderabad appears driven by two broad streams of demand, namely; (a) consumer demand for safe and conveniently packaged drinking water, and (b) growing industrial demand for drinking-water in bulk. About 60% of RO plants in Hyderabad are primarily organized to supply bulk water to pharmaceutical and beverage industries. Industries often find it convenient to outsource drinking water for various reasons, such as scarcity of on-site ground water and cost-effectiveness of outsourcing. On the other hand, many hospitals, educational institutions, clubs, hotels and restaurants have in-house RO plants to meet their needs.
Perspective of RO water quality is linked to intended use. For example; upward adjustment of product TDS or adding back of minerals may be relevant for drinking water (Kozisek, 2005). But minerals in RO product water may interfere with components in finished beverages, thereby affecting stability of finished products. Bulk purified water (BPW) should meet relevant pharmacopoeial specifications for chemical and microbial purity (WHO, 2012). While potable water including potable RO water is the basic requirement for food processing, subtle differences in mineral content of water may be of relevance for specific needs. For example, in case of bakeries, too soft (hardness < 50 mg/L) can limit fermentation creating sticky and loose dough and too hard (> 200 mg/L) water can excessively speed up fermentation causing gluten to be tough (Boada, 2020).
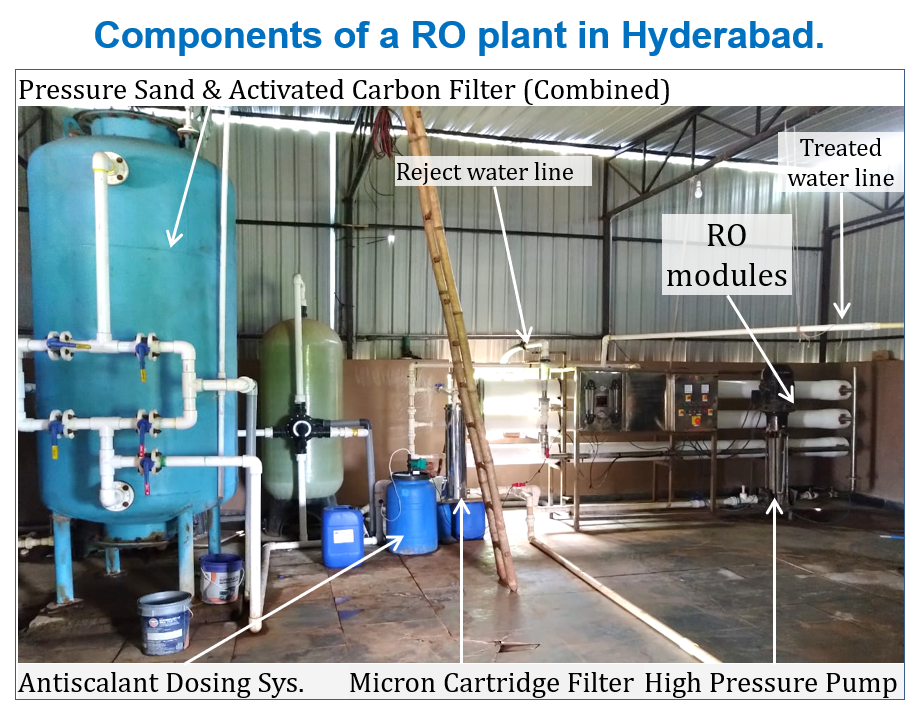
In ROP, we have packaged a good number of physical, chemical and bacteriological, parameters to enable producers and consumers of RO water to test fitness of RO product water for their respective purposes. All physical and sensory parameters are included, except for taste, which can be assessed by the consumer. Chemical parameters include several monovalent (chloride, fluoride, sodium, potassium) and divalent (calcium, magnesium) ions to help assess rejection performance of RO systems. The coliform tests leading up to identification of faecal coliforms and E. coli is essential for determining whether a health risk exists. Heterotrophic plate count (also known as aerobic microbial count) gives an indication of the post-treatment disinfection process. RO water producers use this test along with GPT of feed water to calculate RO-rejection rates.
Sample - Collection, Storage & Transportation:
Follow methods of sampling specified in IS 17614 Part 1:2021 for chemical tests and in IS 1622 : 1981 for bacteriological tests. Use a 1L clean and dry clear or amber colour glass or polypropylene bottle (CBWS / ABWS) for chemical lab sample, and a 250 ml clean sterile bottle (CSBF / SBTD) for bacteriological sample. In case of RO-plant point, collect from the RO product water delivery pipe from which water containers are filled. If you are collecting from acceptance point, collect from bulk water tank delivery pipe or pour from a randomly selected water can. If you care collecting from an in-house RO plant connected to usage points, collect from a tap closer to the point-of-use.
Information About Source, Context, Intended Use & Concerns:
Provide as much detail as you can about the supplier, batch number, bottling date, date of opening of bottle, etc. Mention about intended use of the water, the reason why you are ordering the test, as well as doubts and concerns, if any. These information help in interpretation of test results. Occasionally, the IHS Laboratory may contact you for clarifications and additional information about the source and its environment, to help interpretation of test results.
Test Method & Duration:
Tests are conducted as per appropriate Indian Standard and/or American Public Health Association (APHA) Standard Methods. Test methods in brief for parameters included in this package can be seen at https://www.ihs.org.in/lab/chemlab.html (physical & chemical) and https://www.ihs.org.in/lab/biolab.html (microbial). Depending on duration of bacteriological analysis, and gathering of additional information for interpretation of results; report will be available in 3 to 5 days.



To pick up sample collection bottle and/or schedule collection of samples: Email: ihslab@ihs.org.in with your address and contact telephone; OR WhatsApp: +919848011251; Or Call: 23211013/4.
References:
- IS10500. 2012. Indian Standard Drinking Water Specification. 2nd Revision. New Delhi: Bureau of Indian Standard (BIS); 2012 May, RA2023Amd4.
- IS14543. 2024. Packaged Drinking Water (Other than Packaged Natural Mineral Water) - Specification (3rd Revision). New Delhi: Bureau of Indian Standards (BIS); Indian Standard, 2024 March.
- WHO, 2022. Guidelines for drinking-water quality. 4th Edition, 1st & 2nd addenda. Geneva: WHO, 2022. https://www.who.int/publications/i/item/9789240045064.
- WHO, 2003. Total dissolved solids in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. Geneva: World Health Organization (WHO); 2003. https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/tds.pdf
- Vingerhoeds, et al. 2016. Sensory quality of drinking water produced by reverse osmosis membrane filtration followed by remineralisation. Water Research. 2016 May; 94:42-51. https://doi.org/10.1016/j.watres.2016.02.043/
- Kozisek Frantisek. 2005. Health Risks from Drinking Demineralised Water. Ch-12 in: WHO. Nutrients in Drinking Water. Geneva: World Health Organization (WHO), 2005: 148-63. https://apps.who.int/iris/handle/10665/43403
- NGT. Whether RO plants need to be deployed at all locations irrespective of water quality of raw water. Judgement. New Delhi: National Green Tribunal, Principal Bench; 2019 May 20; OA# 134/2015.
- Boada Alisen, in Tap Score Website. Why Is Water Quality Important in Baking? Berkeley: Simplelab, Inc.; 2020 May 4. https://mytapscore.com/blogs/tips-for-taps/why-is-water-quality-important-in-baking
- WHO. 2012. Annex 2: WHO good manufacturing practices: Water for pharmaceutical use in WHO Expert Committee on Specifications for Pharmaceutical Preparations. Geneva: World Health Organization; Technical Report Series 970, pp. 67-90. https://www.who.int/publications/i/item/WHO_TRS_970
- USFDA. 2014. Reverse Osmosis. Silver Spring, MD, USA: United States Food & Drug Administration (USFDA); 2014 Aug 26; Inspection Technical Guides (ITG). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/reverse-osmosis
