Dialysis water Basic (DLB) tests
Scope:

Physical & Sensory Parameters:
Colour (Appearance & True Colour Units), Odour, pH, Turbidity; & Electrical Conductivity (EC).

Chemical Parameters:
Total chlorine, Total hardness of water (THW), Calcium (Ca) & Magnesium (Mg); Sodium (Na), Potassium (K), Nitrates (NO3); Fluoride & Sulphate (SO4).

Bacteriological Parameters:
Total Viable Count (TVC) Using Tryptic soy agar & 48h incubation at 35-37°C (ISO23500-3).
Total parameters: 5 + 9 + 1 = 15
Rationale:
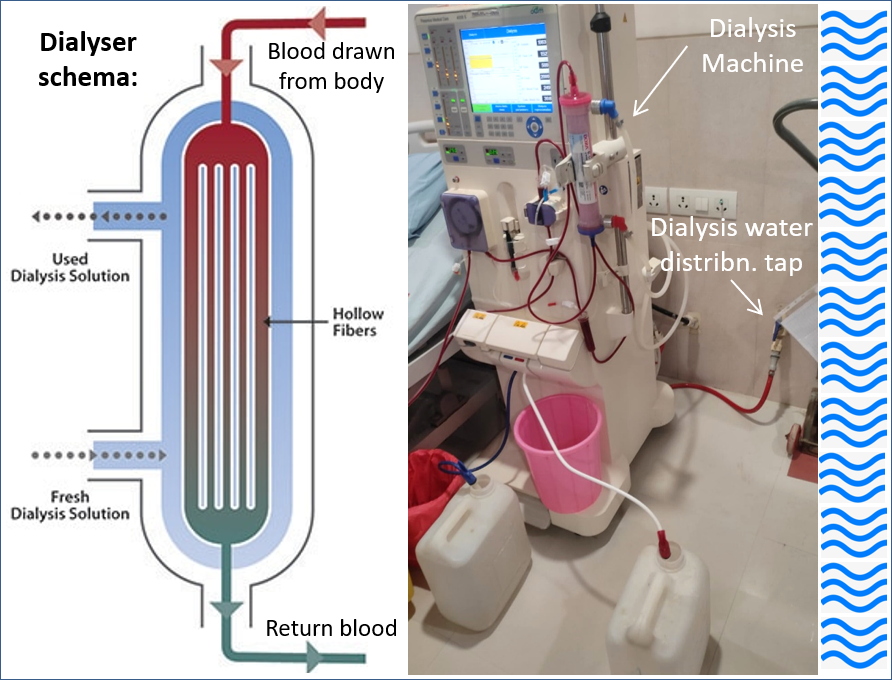
Chronic kidney disease (CKD) prevalence is increasing, all over the world. In India, about 11.5 crore people are living with CKD, as off 2017 (GBD-CKD Collaboration 2020). Haemodialysis is critical for survival of many patients with CKD. During hemodialysis, blood, drawn from a patients body is pumped through a dialyser, inside a semipermeable membrane, surrounded by dialysis solution (dialysate) flowing in the opposite direction. Undesired waste in the blood permeates into the dialysate, and bicarbonate (a needed solute that helps in pH balance) flows from the dialysate into the blood. The clean blood is then returned to patient’s body. Removing the harmful waste, extra salt and fluids help control blood pressure, pH balance, and plasma volume, similar to the results of a functioning kidney (NIDDK, 2018).
Water is used to prepare the dialysate. Haemodialysis can expose the patient to more than 500L of water per week across the semi-permeable dialyser membrane. Compare this with the average weekly water intake of 12-20L, by healthy individuals. Thus, dialysis patients are exposed to about 25 to 40 times the average intake of water. Hence the level of electrolytes, chemical contaminants and trace elements must be much lower than what is allowed for drinking water. Some chemicals are not inherently toxic in nature, but, if present in high enough concentrations, they can cause adverse health effects. Calcium is one such example, where excessive amounts have been associated with renal disease. Microbial contamination of dialysis water and/or dialysate may produce bacteremia and chronic inflammation (Coulliette and Arduino, 2013).
Requirements of water quality for haemodialysis and related therapies as specified in ISO 23500-3 is summarised in the following table.
Summary of dialysis water quality requirements, specified in IS 17646:2021 / ISO 23500-3:2019.
| Sl | Contaminant | Max Conc. mg/L |
|---|---|---|
| Table 1a. Electrolytes | ||
| a | Calcium | 2.0000 |
| b | Magnesium | 4.0000 |
| c | Sodium | 70.0000 |
| d | Potasium | 8.0000 |
| Table 1b. Contaminants with documented toxicity in haemodialysis: | ||
| a | Total chlorine | 0.1000 |
| b | Nitrate-Nitrogen | 2.0000 |
| c | Fluoride | 0.2000 |
| d | Sulfate | 100.0000 |
| e | Aluminium | 0.0100 |
| f | Copper | 0.1000 |
| g | Lead | 0.0050 |
| h | Zinc | 0.1000 |
| Sl | Contaminant | Max Conc. mg/L |
|---|---|---|
| Table 2. Trace elements (Heavy Metals) | ||
| a | Antimony | 0.0060 |
| b | Arsenic | 0.0050 |
| c | Barium | 0.1000 |
| d | Beryllium | 0.0004 |
| e | Cadmium | 0.0010 |
| f | Chromium | 0.0140 |
| g | Mercury | 0.0002 |
| h | Selenium | 0.0900 |
| i | Silver | 0.0050 |
| j | Thallium | 0.0020 |
| 4.3 Microbiological Requirements: | ||
| a | Total viable microbial count (TVC) | < 100 cfu/ml |
| b | BET: Bacterial endotoxin test | < 0.2 EU/ml |
Note: Parameters in italics are included in this, i.e., the DLB test package.
Chlorine or chloramines might have been added to feed water as disinfectants. Dialysis patients should not be exposed to more 0.5mg/L of free chlorine and 0.1 mg/L of combined chlorine/chloramines. To simplify testing, ISO 23500-3 specifies the total chlorine test and assumes that all of it is combined chlorine/chloramine.
Various regional and national professional bodies have developed guidelines regarding dialysis water characteristics such as electrical conductivity, hardness in addition to the specs in ISO23500-3 and recommended frequency of testing. For example; the South Australian haemodialysis guidelines (Whittington et al, 2015) specifies that electrical conductivity (EC) should be <25 µ Siemens/cm and hardness should be less than 20 mg/L. Both these parameters are included in the scope of this test.
Recognising that laboratory facilities and analytical tools to test for trace elements may not be easily available, and to minimise operational constraints, ISO 23500-3, provides for an alternative protocol for demonstration of conformity, based on feed water quality and RO rejection rate. The feed water for dialysis RO plant should be potable and the RO rejection rate, calculated on the basis of electrical conductivity (EC) or total dissolved solids (TDS) must be >90%. Similar alternative provisions for demonstration of compliance are contained in the UK guidelines (Hoenich, et al, 2016) and the South Australian haemodialysis guidelines (Whittington et al, 2015). In order to compute the RO rejection rate, both feed water and product water have to be analysed. Thus, if dialysis-RO feed water (DRF) sample is tested simultaneously, EC results from this (DLB) and the DRF can be used to compute RO Rejection rate.
The South Australian Haemodialysis Guidelines recommend monthly testing of electrolytes & contaminants and annual testing for trace elements. The UK guidelines recommend testing for electrolytes and chemical contaminants once in three months. For microbial characteristics namely TVC and BET, the UK guidelines recommend a monthly interval.
Scope of the dialysis water basic (DLB) tests, include all electrolytes, common chemical contaminants, and the most commonly tested microbial parameter, namely total viable microbial count (TVC). The bacterial endotoxin test (BET) is a separate test package.
Sample - Collection, Storage & Transportation:
Refer methods of sampling specified in IS 3025 part 1 : 1987 for chemical tests and in IS 1622 : 1981 for bacteriological tests. Sample should be representative of the water to be tested and should be collected with utmost care to ensure that no contamination occurs at the time of collection or prior to examination by the laboratory.
Step-1: Gather all that you need for collection of water sample:
A 1000 ml clean and dry polypropylene bottle (CBWS) is required for physical and chemical tests. About 250 ml of water sample collected in a clean sterile bottle (CSB) is required for bacteriological test. In addition to the 2 sample collection bottles (1CSB+1xCBWS), two black or dark colour polythene bags (small garbage bag will do) to minimise exposure of samples to sunlight, ice packs to keep the sample bottles cool during transport and a carry bag for convenient transport.
Step-2: Identify sampling point and time:
Identify a convenient tap from the dialysis water distribution line, preferably inside the dialysis room. As access to dialysis room is usually restricted, seek permission and follow protocol for entry into the dialysis room. Alternatively request a dialysis nurse or other health worker to help collect samples from any of the available dialysis water distribution tap from inside the dialysis room. If none of the taps inside dialysis room is available, then collect from a tap from the dialysis water distribution line, as close to the dialysis area as is feasible. Choose a day when the laboratory is open and collect in the forenoon, so that the sample can reach the laboratory by noon and processing can start on the same day.
Step-3: Collect sample:
Before entering the dialysis area, wash both your hands with soap and water, wipe with a clean towel and let it dry. Put on disposable shoe covers and gloves. Label the sample collection bottles and place it within easy reach, but do not open at this stage. Have ice packs ready. Flush the delivery pipe by letting water out into a bucket for, say 2-3 minutes. Do not touch the flow from delivery pipe. Collect sample for physical and chemical analysis first; then the bacteriological-sample. Grab the bottle in one hand, open and hold the cap in the other hand avoiding to touch inner side of the cap. Place opened bottle mouth under the delivery pipe or spout avoiding direct contact. As the bottle is about to be full, quickly remove the bottle away from water stream and replace cap tightly. Wipe outside of bottle dry with a clean and dry tissue or cloth. Place each bottle inside separate dark colour bags, tie ice packs around each of them and place it in a carry bag for transport to laboratory.
Step-4: Rush to laboratory as soon as possible. Do not store!
Information About Source, Context, Intended Use & Concerns:
Provide as much detail as you can about the sampling point. Mention past tests or date of last sample collection. Gather details regarding source of feed water, simultaneous DRF sample if any, the type of dialysis water distribution system and last date of its disinfection.
Two types, namely direct and indirect feed dialysis water distribution systems are prevalent. In the direct feed water distribution systems, RO plant output passes through an endotoxin filter and is directly connected to the distribution loop delivering provide purified water to the various points of use in the dialysis room. Unused purified water is returned as feed water and is recycled through the RO plant. In the indirect water distribution systems, the purified water from the RO plant is stored in a specially designed holding tank equipped with water-level control devices. These devices interact with the RO plant, turning it off and on as needed and keeping the appropriate water level in the holding tank, so that the tank does not go dry or overfill. The purified water in the holding tank is repressurized by the distribution booster pump, which directs the purified water from the tank through an endotoxin filter before proceeding out to the distribution loop, providing purified water to the various points of use in the dialysis room. Indirect purified water distribution systems return unused purified water back to the holding tank (Kasparek and Rodriguez, 2015).
Test Method & Duration:
Sensory, physical and chemical characteristics of water sample are tested according appropriate parts of the IS3025 and/or American Public Health Association (APHA). Microbial parameter, namely TVC is estimated using Tryptic Soy Agar, and 48h incubation at 35 °C to 37 °C as specified in ISO 23500-3. Report will be available in 4 to 6 days.



To pick up sample collection bottle and/or schedule collection of samples: Email: ihslab@ihs.org.in with your address and contact telephone; OR WhatsApp: +919848011251; Or Call: 23211013/4.
References:
- Coulliette Angela D and Arduino Mathew J. Hemodialysis and Water Quality. Seminars in Dialysis. 2013 Jul-2013 Aug 31; 26(4):427-38. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4596525/
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2020 Feb 29; 395(10225):709-733. https://pubmed.ncbi.nlm.nih.gov/32061315/
- Hoenich Nic; Mactier Robert; Boyle Gerard; et al. Guideline on water treatment facilities and water quality for haemodialysis and related therapies. UK Renal Assoc. & Assoc. of Renal Technologists; 2016. https://www.renaltech.net/uploads/1/3/6/4/136400/guideline_on_water_treatment_systems_dialysis_water_and_related_therapies__jan_2016.pdf
- IS 1622: 1981. Indian Standard Methods of Sampling and Microbiological Examination of Water. New Delhi: Bureau of Indian Standard (BIS); Indian Standard, IS1622 - 1981, Reaffirmed 1996, Amended 2003. https://law.resource.org/pub/in/bis/S02/is.1622.1981.pdf
- IS 3025 Part 1: 1987. Methods of Sampling and Test (Physical and Chemical) for Water and Wastewater. Part 1 Sampling. New Delhi: Bureau of Indian Standard (BIS); Indian Standard. Reaffirmed 2003. https://law.resource.org/pub/in/bis/S02/is.3025.01.1987.pdf
- IS 17646 (Part 3) : 2021 Preparation and quality management of fluids for haemodialysis and related therapies Part 3 Water for haemodialysis and related therapies. Identical under dual numbering (ISO 23500-3 : 2019).
- ISO 23500-3. 2019. Preparation and quality management of fluids for haemodialysis and related therapies — Part 3: Water for haemodialysis and related therapies. Geneva, Switzerland: International Standards Organization (ISO); 2019 Feb; ISO 23500-3:2019.
- Kasparek Ted and Rodriguez Oscar E. What Medical Directors Need to Know about Dialysis Facility Water Management. Clinical Journal of the American Society of Nephrology. 2015 Jun 5; 10(6):1061-1071.https://pubmed.ncbi.nlm.nih.gov/25979976/
- NIDDK. Hemodialysis. Bethesda, MD, USA: US National Institutes of Health (NIH) - National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); 2018 Jan; Health Information. https://www.niddk.nih.gov/health-information/kidney-disease/kidney-failure/hemodialysis
- Whittington Tiffany; Donnelly Fiona; Passaris George; et al. South Australian Haemodialysis Guidelines: Routine Water Testing and Reverse Osmosis Monitoring. 2015 Jun.https://www.sahealth.sa.gov.au/wps/wcm/connect/bd096e004a4622f78af7cfb0cfc4074a/Haemodialysis_Routine+Water+Testing+and+Reverse+Osmosis+Monitoring_V3_1.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-bd096e004a4622f78af7cfb0cfc4074a-og8xKiH
- WHO. 2011. Guidelines for drinking-water quality. Fourth Edition. Geneva: 2011. https://www.who.int/publications/i/item/9789241549950
