Environmental Monitoring of Urban Lakes in Hyderabad Metropolitan Development Area (HMDA).
Urban Lake Water Quality Monitoring in HMDA Area.
Urban water bodies play a key role in sustaining healthy environment in cities and moderating adverse impacts of high population density and industrialization. Unfortunately, the scarcity of land, pressure of development and inadequate investments tend to threaten the quality of water and sometimes the very existence of urban lakes. Nurturing and protection of urban water bodies is important for healthy neighbourhoods and sustainable cities. Smart cities rely on field data and information gathered from multiple sources to manage urban ecosystem, improve operations and enhance quality of life.
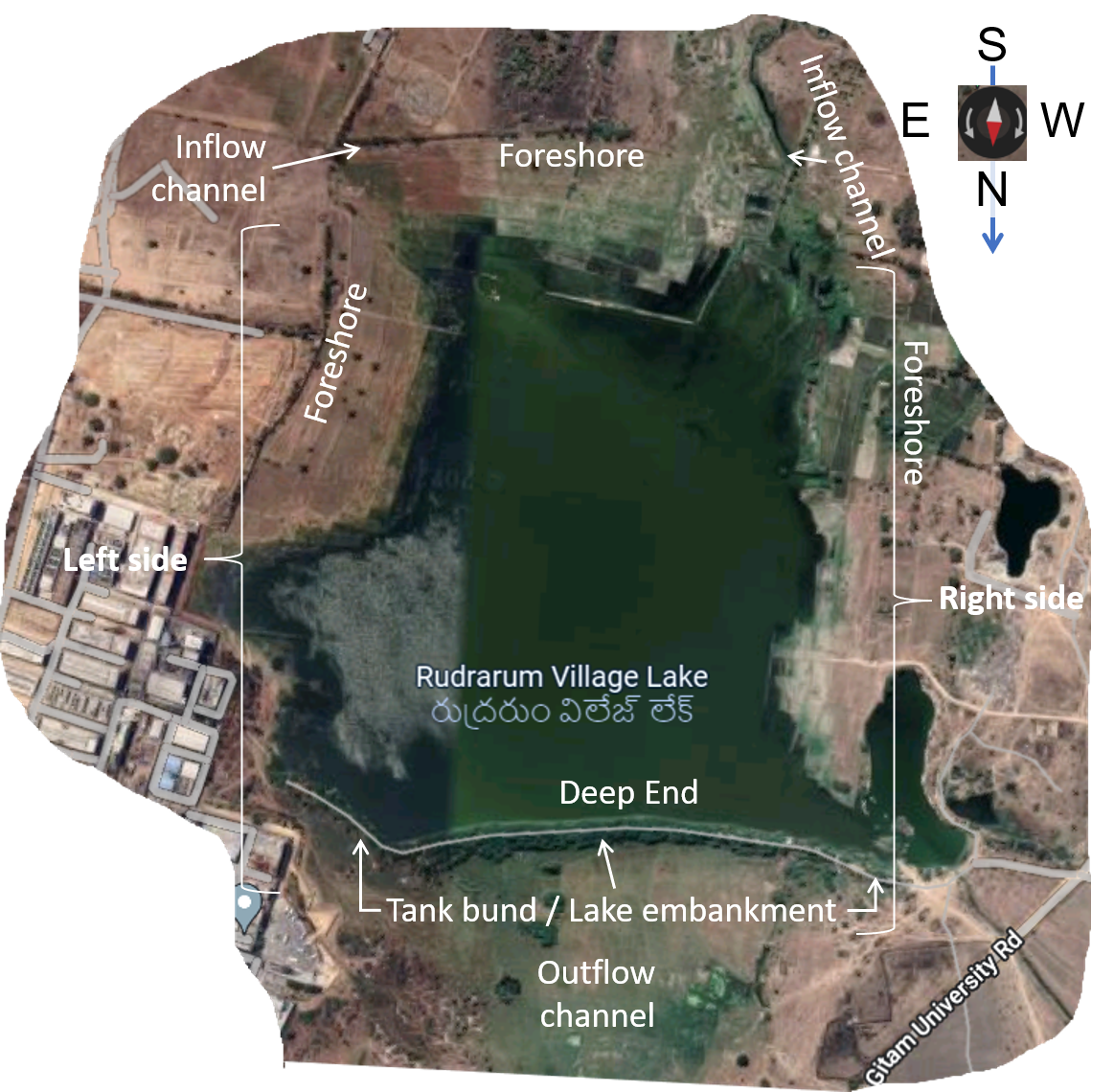
Urban water bodies in the Hyderabad metropolitan area consist of many small to medium sized lakes and irrigation tanks that were built primarily for irrigation and/or fisheries. Many of these water bodies were part of cascading chain tank systems designed to conserve water. Urbanisation poses a challenge for these water bodies. Protection and maintenance of tanks for irrigation takes a backseat due to changing land use from agriculture to residential, institutional and industrial purposes. So, yesteryears irrigation tanks and lakes have become urban lakes and water bodies. Though relatively small in size, these urban lakes are important environmental, social, and economic assets. Juxtaposed with conventional utility for irrigation and fisheries, new uses such as recreation, tourism, and water sports appear. Concomitantly, urban water bodies face existential threats and new challenges, such as the vulnerability of encroachments and pollution.
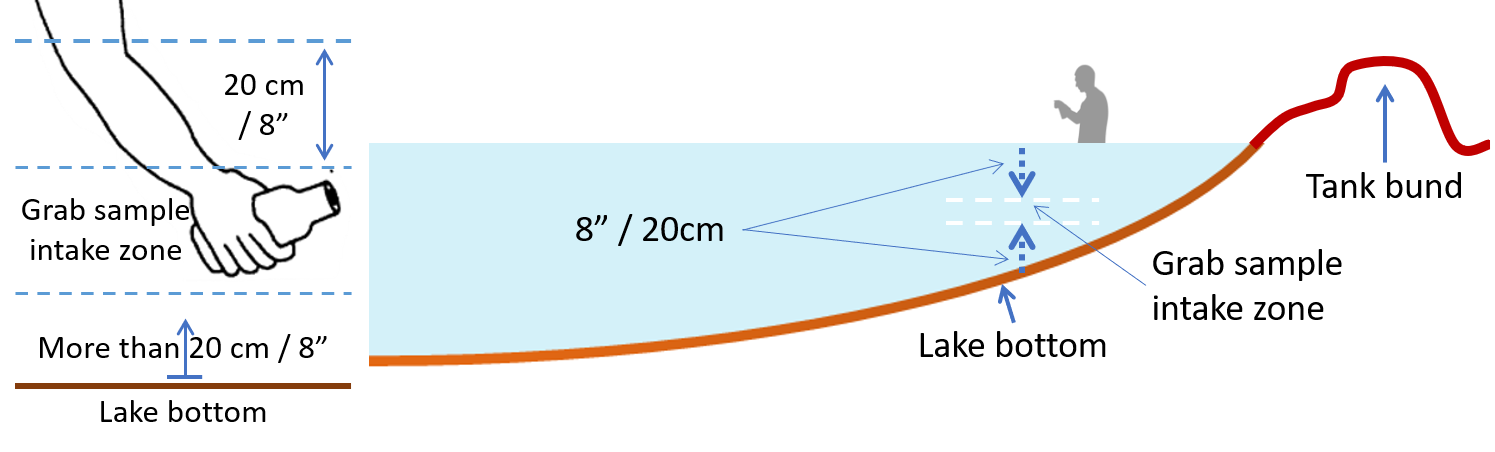
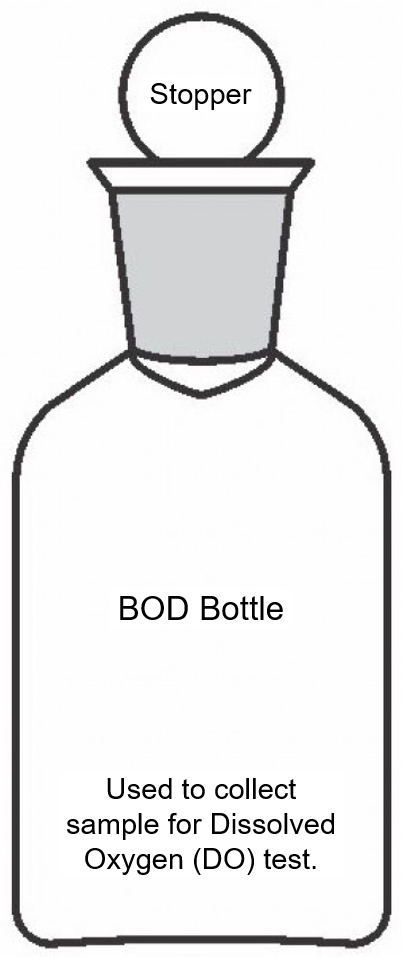
Field observation of the urban water bodies and monitoring of their water quality provides a basis and required data for effective protection and nurturing of the water bodies for contemporary public uses and to improve overall environment of the city. To facilitate monitoring of water quality in water bodies in and around Hyderabad, the IHS has developed a Tank/Lake Water Sample Collection Guide. The Institute is monitoring water quality along with field observation of HMDA lakes, assigned by the Telangana State Pollution Control Board from time to time.